The market based price for Cobenfy is worth it
TURN THE PAGE
A Leerink Center for Pharmacoeconomics (CPE) Exclusive found the market-based price for Cobenfy™ to be less than the societal benefit it provides even under conservative assumptions. This is great news for society and is something to celebrate.
This marks the first CPE Exclusive we have released. CPE was launched with the goal of evaluating the societal impact of healthcare innovations, and we intend to release CPE Exclusives regularly to help explain the impact an innovation has on patients, caregivers, the health system, and society as a whole.
Our first CPE Exclusive comes on the heels of a recently published peer-reviewed manuscript that details current best practices to value the societal impact of medicines. I was honored to be a co-author, alongside 11 other health economists, of this user guide that describes the methods and parameters to incorporate societal impacts within economic models and cost-effectiveness analyses. For many years, the field of health economics has been talking about the need to include these novel value elements within cost-effectiveness analyses. With this user guide, we can now move from talking about novel value elements to actually measuring them.
The methods we apply in our CPE Exclusives closely follow the framework proposed in the user guide. Our first CPE Exclusive evaluates the societal impact of Cobenfy. The U.S. Food and Drug Administration approved Cobenfy (xanomeline and trospium chloride) on September 26th, 2024. The approval represented the first significant innovation in the approach used to treat schizophrenia in decades. Cobenfy is unique in that it has been shown to reduce schizophrenia symptoms without weight gain. This weight gain advantage over existing agents has massive implications for individuals living with schizophrenia, their caregivers, the health system, and society.
We anticipate Cobenfy will have a positive impact on 23 of 24 value elements and that it could be cost saving to society if you believe it is not associated with weight gain and is associated with downstream productivity gains. In the waterfall diagram below, we present the incremental cost-effectiveness ratio for Cobenfy as compared to oral second-generation antipsychotics using conventional cost-effectiveness analysis methods and the impact quantifying each novel domain of value has on the incremental cost-effectiveness ratio.
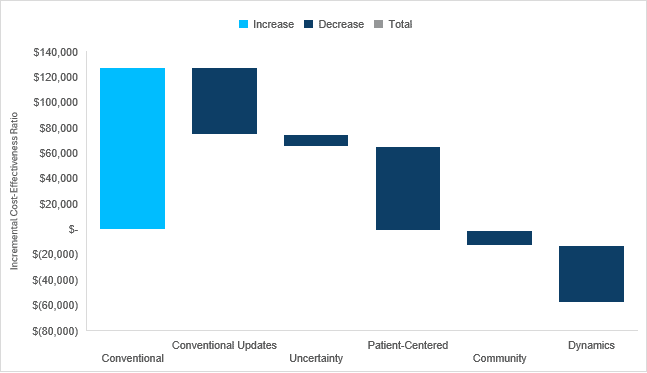
Check out our full report-to understand the specific impact Cobenfy may have on each domain of value, how it may perform under conventional and generalized cost-effectiveness analysis frameworks, and why our estimates are likely conservative.
WE DID IT AGAIN
Expensive branded drugs will likely become cheap generics that can benefit society long after their patent protection period. This is important for policy making and for public perception around drug pricing. It also really matters for cost-effectiveness analysis as can be seen in our analysis of Cobenfy (see the “Turn the Page” section). Adding the dynamic domains in the generalized cost-effectiveness analysis for Cobenfy made the cost-effectiveness estimate even more favorable by reducing the incremental treatment costs.
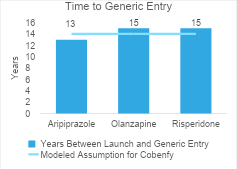
For Cobenfy, we assumed generic competition would enter the market after 14 years and would result in Cobenfy’s price dropping to two times its cost of goods sold. We estimated the post-loss of exclusivity price as $146 per year. These future cost estimates are supported by real-world data on generic pricing and time to generic entry for second-generation antipsychotics.
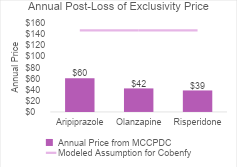
The first graph shows the current annual prices (from the Mark Cuban Cost Plus Drug Company) for the second-generation antipsychotics that represented the comparator basket in our analysis. These all have generic competition today. The annual post-loss of exclusivity price we assumed for Cobenfy was higher than all of these (as indicated by the purple line). This could be an overestimate but because Cobenfy is a twice-daily regimen, there could be twice as many pills per year as compared to the other regimens which could result in a higher annual price if the cost was based on its cost of goods sold.
The second graph shows the time between launch and generic entry for the second-generation antipsychotics. Generic versions entered between 13 and 15 years after launch. The time to generic entry we assumed for Cobenfy was in line with these estimates (as indicated by the blue line).
SAD BUT TRUE
Cost-effectiveness analyses rarely consider the impact an innovation has on patient productivity. As demonstrated within our analysis for Cobenfy (see the “Turn the Page” section), accounting for patient productivity can have a dramatic impact on the cost-effectiveness estimate. A study by Dr. David Kim and colleagues analyzed the Tufts Medical Center’s Cost-Effectiveness Analysis Registry and found that although patient productivity was the most common non-health consequence included in cost-effectiveness analyses, it was only included in 12% of cost-effectiveness analyses.
The study by Kim and colleagues is a few years old, so there is a chance the number is slightly higher now—at least I would hope so given the Second Panel on Cost-Effectiveness in Health and Medicine was published in 2016 and recommended the incorporation of productivity in the societal perspective of cost-effectiveness analyses. However, even if the number of cost-effectiveness analyses that consider patient productivity is higher today (which would be excellent), the impact is likely greatly underestimated even in those studies that consider an innovation’s impact on patient productivity.
It is common in economic models to program productivity as productivity losses, more specifically time missed from work. For example, assume an individual misses 21 days of work for a COPD exacerbation. This would commonly be programmed in the economic model as a productivity loss—assigning 21 days of missed work per exacerbation. If the intervention resulted in fewer exacerbations, then it would result in less time missed from work. However, it is not as common for economic models to consider productivity gains due to life extension, productivity associated with non-market production, or productivity associated with household production.
Drs. Jiao and Basu developed an approach that can easily be implemented in economic models to capture productivity associated with labor, education, caring for household members, caring for non-household members, time seeking medical care, and volunteering in periods of baseline survival and in life extension. In their paper, they applied their approach to a previous economic model they had built. When only including formal labor time in baseline survival, the cost-effectiveness estimate only changed by -1%. However, when including all types of productivity in baseline survival and in life extension, the cost-effectiveness estimate changed by -27%.
Cost-effectiveness analyses must be more consistent in the incorporation of productivity in the societal perspective, and the field needs to align on what counts as “checking the box” to determine if an analysis includes patient productivity.
Disclosures
The Center for Pharmacoeconomics (“CPE”) is a division of MEDACorp LLC (“MEDACorp”). CPE is committed to advancing the understanding and evaluating the economic and societal benefits of healthcare treatments in the United States. Through its thought leadership, evaluations, and advisory services, CPE supports decisions intended to improve societal outcomes. MEDACorp, an affiliate of Leerink Partners LLC (“Leerink Partners”), maintains a global network of independent healthcare professionals providing industry and market insights to Leerink Partners and its clients. The information provided by the Center for Pharmacoeconomics is intended for the sole use of the recipient, is for informational purposes only, and does not constitute investment or other advice or a recommendation or offer to buy or sell any security, product, or service. The information has been obtained from sources that we believe reliable, but we do not represent that it is accurate or complete and it should not be relied upon as such. All information is subject to change without notice, and any opinions and information contained herein are as of the date of this material, and MEDACorp does not undertake any obligation to update them. This document may not be reproduced, edited, or circulated without the express written consent of MEDACorp.
© 2025 MEDACorp LLC. All Rights Reserved.
Disclosures
The Center for Pharmacoeconomics (“CPE”) is a division of MEDACorp LLC (“MEDACorp”). CPE is committed to advancing the understanding and evaluating the economic and societal benefits of healthcare treatments in the United States. Through its thought leadership, evaluations, and advisory services, CPE supports decisions intended to improve societal outcomes. MEDACorp, an affiliate of Leerink Partners LLC (“Leerink Partners”), maintains a global network of independent healthcare professionals providing industry and market insights to Leerink Partners and its clients. The information provided by the Center for Pharmacoeconomics is intended for the sole use of the recipient, is for informational purposes only, and does not constitute investment or other advice or a recommendation or offer to buy or sell any security, product, or service. The information has been obtained from sources that we believe reliable, but we do not represent that it is accurate or complete and it should not be relied upon as such. All information is subject to change without notice, and any opinions and information contained herein are as of the date of this material, and MEDACorp does not undertake any obligation to update them. This document may not be reproduced, edited, or circulated without the express written consent of MEDACorp.
© 2025 MEDACorp LLC. All Rights Reserved.
Disclosures
The Center for Pharmacoeconomics (“CPE”) is a division of MEDACorp LLC (“MEDACorp”). CPE is committed to advancing the understanding and evaluating the economic and societal benefits of healthcare treatments in the United States. Through its thought leadership, evaluations, and advisory services, CPE supports decisions intended to improve societal outcomes. MEDACorp, an affiliate of Leerink Partners LLC (“Leerink Partners”), maintains a global network of independent healthcare professionals providing industry and market insights to Leerink Partners and its clients. The information provided by the Center for Pharmacoeconomics is intended for the sole use of the recipient, is for informational purposes only, and does not constitute investment or other advice or a recommendation or offer to buy or sell any security, product, or service. The information has been obtained from sources that we believe reliable, but we do not represent that it is accurate or complete and it should not be relied upon as such. All information is subject to change without notice, and any opinions and information contained herein are as of the date of this material, and MEDACorp does not undertake any obligation to update them. This document may not be reproduced, edited, or circulated without the express written consent of MEDACorp.
© 2025 MEDACorp LLC. All Rights Reserved.

